Research
Our projects
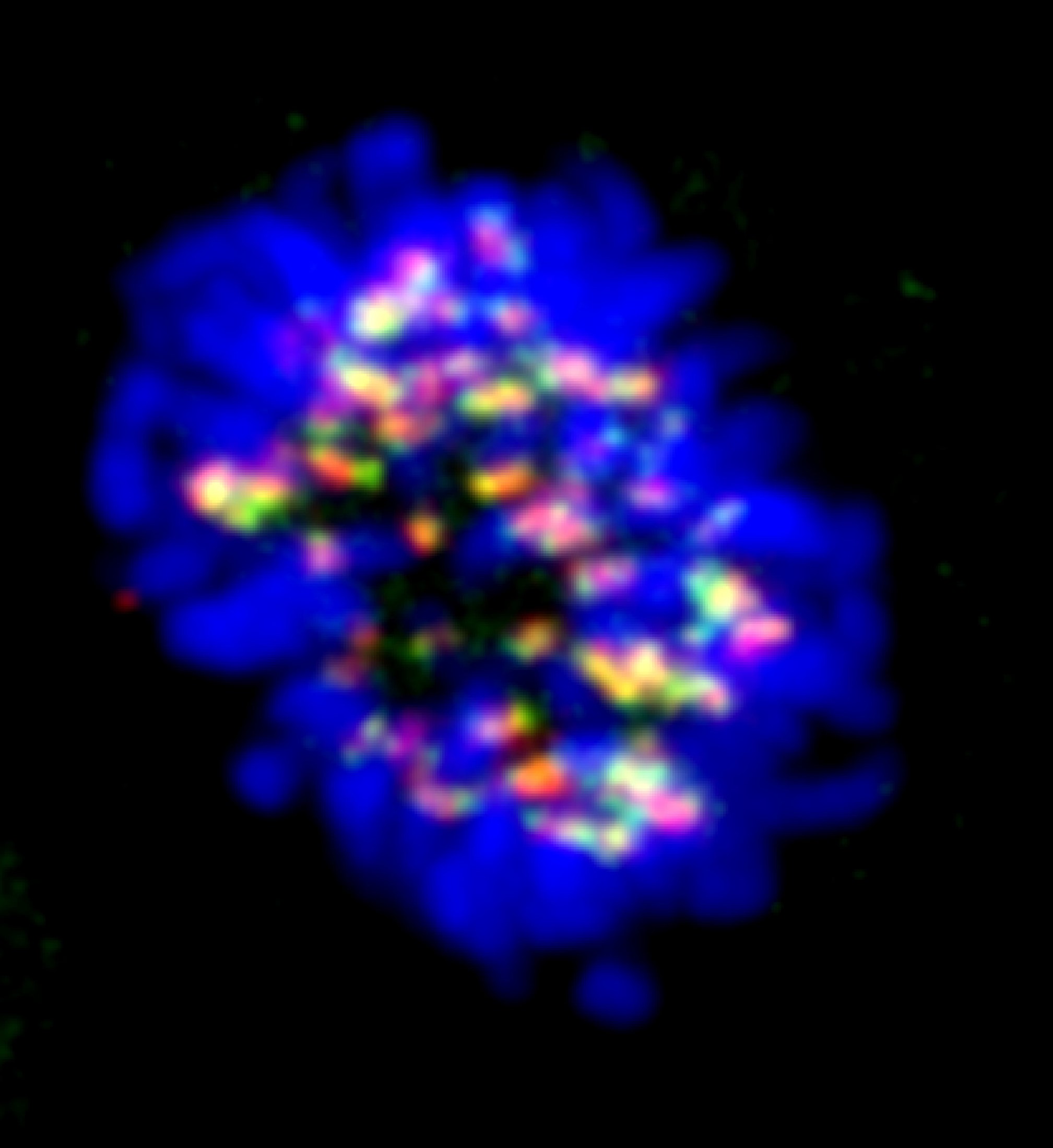
For successful mitotic cell division, correct (bipolar) attachment of all duplicated chromosomes to spindle microtubules during prometaphase and metaphase is followed by chromosome segregation (anaphase), cytoplasmic division (cytokinesis) and abscission (the final cut of the narrow intercellular cytoplasmic canal to release the two daughter cells) in a well-choreographed manner. Furthermore, the cell has surveillance (“checkpoint”) mechanisms that monitor the above mitotic processes and can delay mitotic progression in the case of misattached or missegregated chromosomes until the problem is fixed. Our lab is particularly interested in understanding how such mechanisms function on the molecular level and their significance for maintaining chromosomal stability .
The mitotic spindle checkpoint
The mitotic spindle checkpoint protects against chromosome missegregation and aneuploidy by delaying sister chromatid separation in the presence of unattached or improperly attached kinetochores. For this purpose, spindle checkpoint proteins accumulate on unattached or mis-attached kinetochores to prevent activation of the anaphase-promoting complex/cyclosome (APC/C) and delay anaphase onset. The DNA damage response on the other hand, is a complex network of signaling pathways that coordinates cellular reactions, ranging from cell cycle arrest and DNA damage repair to the induction of senescence or apoptosis, to DNA lesions.
· Studies ranging from the 1950s until recently had suggested that the DNA damage response and the mitotic cell division are unrelated processes with the former preserving genome stability during interphase and the cell division machinery ensuring accurate distribution of the genetic material to the two daughter cells. Findings from our lab were perhaps the first to challenge this view and it is now becoming recognized that DNA damage response proteins also play essential roles in mitosis in the absence of damaged DNA, indicating functional interactions between these two essential mechansms that maintain genome stability.
·
More specifically, we showed that the DNA damage response
kinases Chk1 and Chk2 promote correction of misattached kinetochore-microtubules and spindle
checkpoint signaling by phosphorylating the Aurora B kinase, an important
regulator of mitotic cell division that forms the catalytic core of the
chromosomal passenger complex (CPC), in prometaphase. We also identified serine
331 (S331) as the conserved Chk1/Chk2-site on Aurora B and determined how phosphorylation
of this site promotes Aurora B catalytic activity.
· We also demonstrated that the endosomal sorting complex required for transport (ESCRT) protein Chmp4c promotes optimal chromosome alignment and segregation by interacting with kinetochore-microtubules and by promoting localization of the RZZ (Rod-Zw10-Zwilch) spindle checkpoint complex to unattached kinetochores. These findings identify unexpected connections between the membrane remodeling and mitotic machineries that remain to be fully explored.
The abscission checkpoint
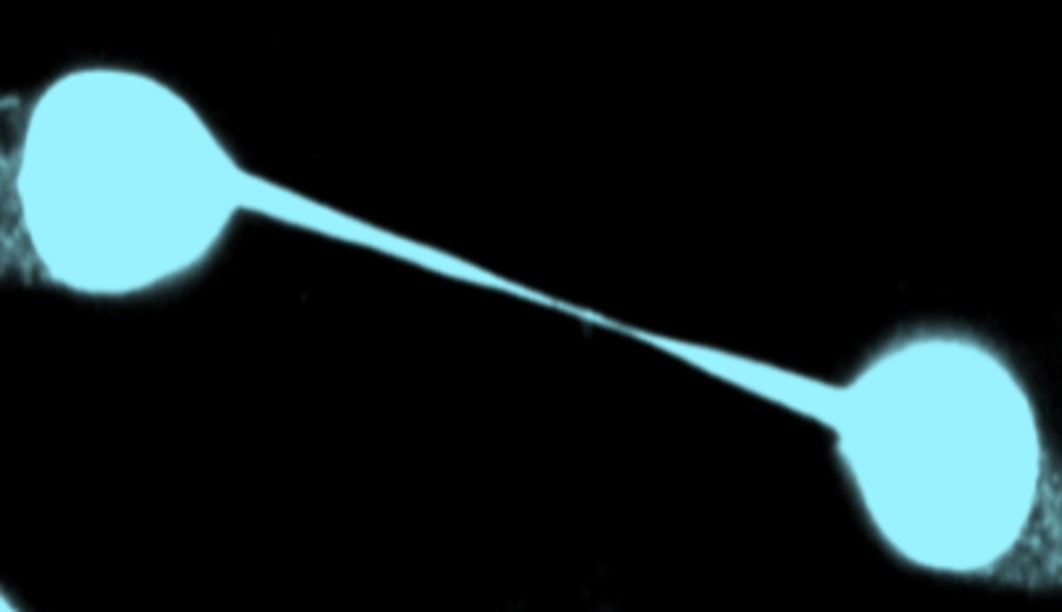
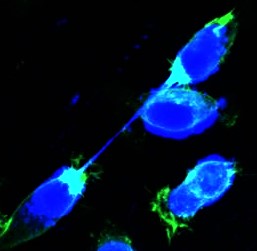
Incompletely segregated DNA can lead to formation of chromatin bridges, i.e. strings of chromatin connecting the anaphase poles or daughter nuclei, that are associated with chromosomal instability in human tumours and tumourigenesis in mouse models. In the presence of chromatin bridges, human cells delay abscission to prevent chromatin breakage and this “abscission checkpoint” is dependent on Aurora B catalytic activity at the midbody.
· Our lab has identified Cdc-like kinases (Clks; kinases with previously known roles in regulating alternative splicing) as upstream regulators of Aurora B in the abscission checkpoint, through Clk-mediated Aurora B-S331 phosphorylation.
· We also showed that Chk1 has an indirect role in protecting cells against chromatin bridges, by stabilizing BLM helicase to facilitate resolution of DNA replication intermediates that can lead to DNA bridges.
· We also identified a novel ATM-Chk2-INCENP pathway that relays chromatin bridges to the CPC to impose the abscission checkpoint and prevent chromatin bridge-breakage in cytokinesis in human cells. We are now further investigating the molecular "sensors" involved.
Actin patches
In cytokinesis with chromatin bridges, cells retain accumulations of polymerized actin (“actin patches”) at the base of the intercellular canal to prevent chromatin breakage. We demonstrated that formation of actin patches is dependent on the catalytic activity of the non-receptor tyrosine kinase Src at the bases of the intercellular canals. We also showed that Chk1 phosphorylates Src on serine 51 to promote Src catalytic activity and that this phosphorylation is required for actin patch formation and stable chromatin bridges in cytokinesis. We have recently identified additional actin-remodeling proteins that participate in the formation of actin patches and are investigating the molecular mechanisms involved.
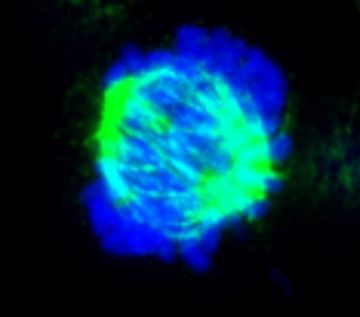
The mitotic spindle
The mitotic spindle is a microtubule-based apparatus that mediates segregation of sister chromatids to the two daughter cells. Errors in spindle formation can lead to chromosome mis-segregation and aneuploidy that are associated with human genetic syndromes, cancer predisposition, or birth defects. As a result, how the mitotic spindle assembles and performs its functions is a matter of intense investigation.
· We have identified novel proteins that promote spindle microtubule polymerization and optimal spindle formation and are investigating how they function. Because spindle disruption by microtubule poisons is a valid anticancer strategy, inhibition of these newly identified proteins may enhance the efficacy of tumour cell killing by microtubule drugs and improve cancer therapy.
Funding our research
We are grateful to the following funding bodies for supporting our work